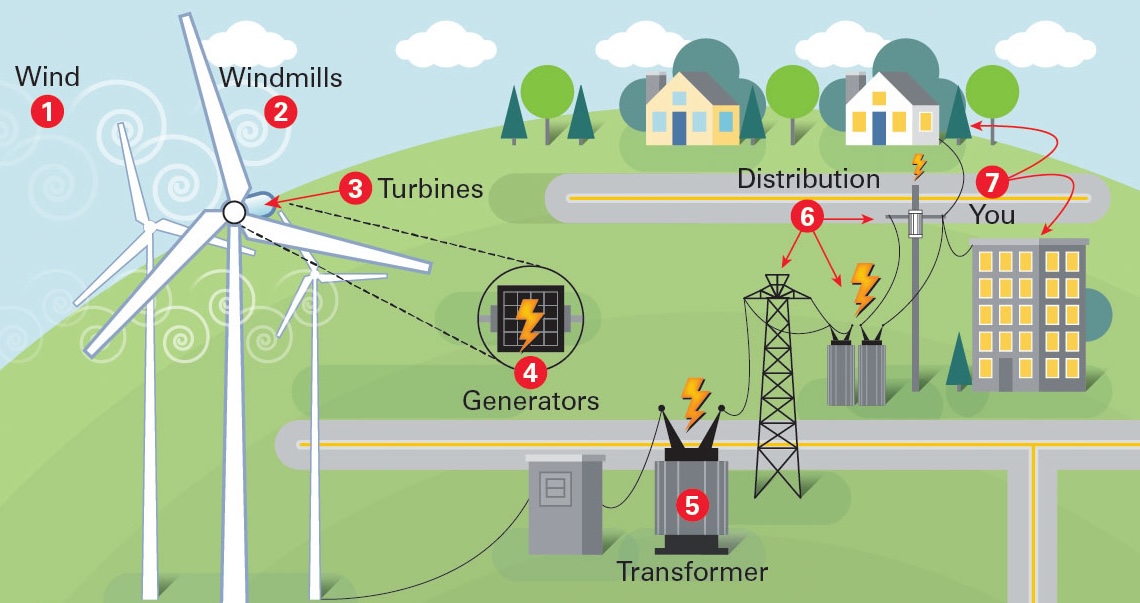
Scientists Use Hydrogen Nanoreactors to Engineer Bacteria That Creates H2
Science News Breakthrough in Green Hydrogen Technology at the University of Oxford Researchers at the University of Oxford have unveiled…

Science News Breakthrough in Green Hydrogen Technology at the University of Oxford
Researchers at the University of Oxford have unveiled a significant breakthrough in the ongoing quest for sustainable energy. Utilizing bio-engineered bacteria referred to as “hydrogen nanoreactors,” their team has developed an innovative method to produce green hydrogen from water and sunlight. This novel approach promises not only to lower the environmental impact of hydrogen production but also to make the process more affordable and safe.
“This marks a significant step forward in the pursuit of a robust and efficient biocatalyst,” remarked lead author Professor Wei Huang. He emphasized the advantages of the technology, including its safety, cost-effectiveness, and potential to boost long-term economic viability.
The Science Behind Hydrogen Nanoreactors
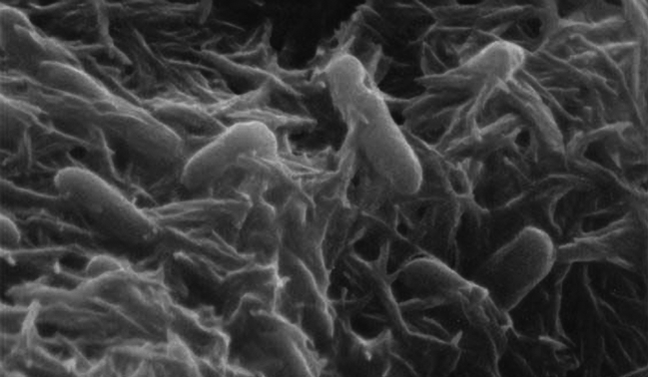
At the heart of the breakthrough is a species of bacteria, Shewanella oneidensis, genetically modified to amplify its natural ability to produce hydrogen. Known for being “electroactive,” this bacterium can transfer electrons to and from solid surfaces. Researchers took advantage of this property by engineering the bacteria to concentrate essential components—electrons, protons, and the hydrogenase enzyme—in a specific cellular compartment called the periplasmic space, located between its inner and outer membranes.
But the ingenuity doesn’t stop there. To enhance efficiency further, a light-activated electron pump was introduced into the bacteria. This pump harnesses energy from sunlight to move protons into the periplasmic space, effectively supercharging the reaction. Additionally, nanoparticles of reduced graphene oxide and ferric sulfate were added to promote electron transfer. With these innovations in place, the hydrogen production yield increased tenfold compared to the unmodified bacteria.
The team has also suggested that this system could one day be scaled up in the form of “artificial leaves,” where sunlight-activated bacterial coatings would continuously produce hydrogen. Imagine a field of these bio-engineered leaves—a profound vision of how biotechnology could harness natural processes to generate renewable energy on a large scale.
Here’s how it works step by step:
-
Electron and Proton Concentration
Within the bacteria, key elements needed for hydrogen production—such as electrons and protons—are concentrated in a confined area called the periplasmic space. This space exists between the inner and outer membranes of the bacterial cell, creating the perfect environment for hydrogen production. -
Light-Activated Electron Pump
To make the process more energy-efficient, scientists integrate a light-activated electron pump into the bacteria. This component harnesses sunlight to generate the energy needed to drive protons into the periplasmic space, creating the conditions for hydrogen production. -
Enhanced Materials
Researchers have also introduced nanoparticles of reduced graphene oxide and ferric sulfate to improve electron transfer within the system. These materials facilitate the movement of electrons, ensuring that the bacteria produce hydrogen fuel at higher yields. -
Overexpression of Hydrogenase Enzyme
A crucial enzyme, hydrogenase, plays a central role in this process. By overexpressing this enzyme within the bacteria, the researchers ensure that the hydrogen production reaction occurs more frequently and efficiently.
The result of these modifications is a tenfold increase in hydrogen output compared to unmodified bacteria. This innovative method allows for the efficient conversion of water and sunlight into clean, renewable hydrogen fuel.
Why This Is a Game-Changer for Green Hydrogen
One of the primary challenges in the green hydrogen industry has been the reliance on costly renewable electricity and materials to split water molecules into hydrogen and oxygen. Until now, industrial hydrogen production largely relied on fossil fuels, resulting in high carbon emissions—ranging from 11.5 to 13.6 kilograms of CO2 for every kilogram of hydrogen produced.
The University of Oxford’s research could dramatically shift this narrative. By using bio-engineered bacteria, this method not only eliminates the need for carbon-heavy processes but also makes use of renewable solar energy. This approach aligns perfectly with long-term climate goals, particularly the transition to net-zero emissions.
Beyond its environmental benefits, the new system offers the promise of reduced production costs. The simplicity of bacterial cultivation—coupled with the use of natural and renewable starting materials—means this technology could be more economically viable compared to existing methods.
Recent Developments and Global Implications
This latest advancement builds on Oxford’s extensive track record in biotech and clean energy innovation. Earlier this year, researchers at the university made headlines with improvements in the efficiency of organic solar cells—another leap forward in the broader renewable energy sector. These combined accomplishments underline Oxford’s commitment to addressing global energy challenges through science and collaboration.
Globally, the stakes for green hydrogen have never been higher. Both public and private sectors have recognized hydrogen as a critical tool for reducing emissions in industries that are hard to electrify, such as aviation, shipping, and heavy manufacturing. However, expanding green hydrogen access has often been hindered by cost and infrastructure barriers. Oxford’s approach could be the catalyst needed to overcome these challenges.
When Will We See This Technology Applied?
While the research is still in its developmental stages, the potential for real-world application is drawing significant attention. Scaled-up production of hydrogen nanoreactors or artificial leaves could take several years, depending on factors like funding, regulatory approval, and industrial partnerships. Some experts predict that pilot implementations could emerge within five to ten years, with more widespread adoption becoming feasible by 2040.
That said, small-scale applications, such as prototypes for localized hydrogen generation, could emerge sooner. For example, rural regions or off-grid communities might benefit from compact versions of this system, leveraging its ability to operate sustainably and independently of centralized energy infrastructure.
How We Can Use This Technology Now
Although widespread adoption may be years away, researchers and policymakers can already explore ways to integrate hydrogen nanoreactors into existing green energy initiatives. Laboratory-scale systems can currently be used to test water-splitting in controlled environments, gathering valuable data to refine the technology further. Meanwhile, partnerships with industries like transportation or energy storage could pave the way for early adoption.
For readers, breakthroughs like this serve as powerful reminders of the importance of sustained investment in clean energy innovation. Green hydrogen has the potential to transform how we power our cities, industries, and even our cars. It’s not just a vision for the future—it’s a tangible pursuit that brings us one step closer to a cleaner, more sustainable world.
By blending biology with clean energy principles, Oxford’s research offers something truly compelling. It’s a glimpse into a future where science and nature collaborate, providing solutions that address both environmental and economic challenges. While we wait for large-scale implementation, every small step—even one powered by bacteria—is a leap in the right direction.
What's Your Reaction?